
As a subsidiary of International Drug Development (IDD), BVP strengthens the group’s capabilities by adding a dedicated pharmaceutical exploitation and commercial platform to IDD’s renowned expertise in development and regulatory consulting.
Together, the group delivers a seamless, end-to-end solution to support your product’s journey to market.
Headquartered in Paris, BVP is a European-based company led by a team of seasoned professionals with over 30 years of experience in the pharmaceutical industry.
Press release dated January the 6th, 2026


The redesigned website reflects IDD’s commitment to providing high-end, tailored solutions across the entire drug value chain. Key features of the new site include:
- In-depth service descriptions: A clear overview of IDD’s core services, including asset pre-approval, medicines post-approval, and business platform services.
- Streamlined navigation: An intuitive design that makes it easy for visitors to find the information they need.
- Modern and responsive design: A seamless experience across all devices.
“We are thrilled to launch our new website and provide our clients and partners with a more engaging and informative online experience,”.
“This new site is a testament to our ongoing commitment to excellence and innovation in the pharmaceutical consulting industry.”
The new website will be regularly updated with news, insights, and company updates. Visitors are encouraged to explore the site and learn more about IDD’s services.
First, Clarisse Ribeiro, who joined in MM/2025, holds a degree in [to be completed], and specializes in [to be completed].
Next, Mohamed ALILI, a Doctor of Pharmacy since 2020 in Algeria, is currently completing an internship to validate his degree in France. He is involved in Quality Assurance and Regulatory Affairs under the direct supervision of Anne Walter.
Finally, we welcomed a second intern in May 2025. Swann Walch is completing his fifth-year pharmacy internship under the supervision of Stéphane Simon, contributing to business development and the integration of AI into company processes.
These new additions reflect the energy and momentum of both companies, their appeal to young talent, and their commitment to excellence, innovation, and knowledge sharing.
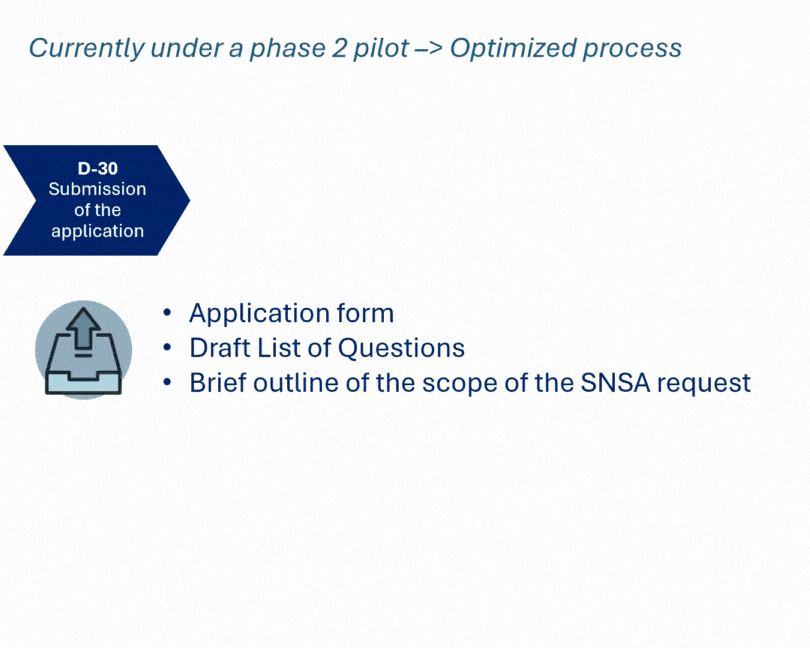
Developing a new medicinal product often involves navigating complex regulatory expectations. We recently supported a client through the SNSA procedure, engaging both Dutch and Belgian authorities in a joint scientific advice meeting.
This coordinated approach enabled a comprehensive review of CMC, non-clinical, and clinical aspects—particularly where existing guidelines lacked clarity. The outcome: a clearer, more confident development path for a complex product, aligned with regulatory expectations from the outset.
🔗 Learn more about SNSA
– Anne Walter



We had the pleasure of attending RAPS Euro Convergence 2024 in Brussels, where regulatory innovation and collaboration were at the heart of the discussions. Key topics included the upcoming EU pharma reform, access to medicines, and digital transformation. A valuable moment to connect with peers and reflect on the future of regulatory affairs.