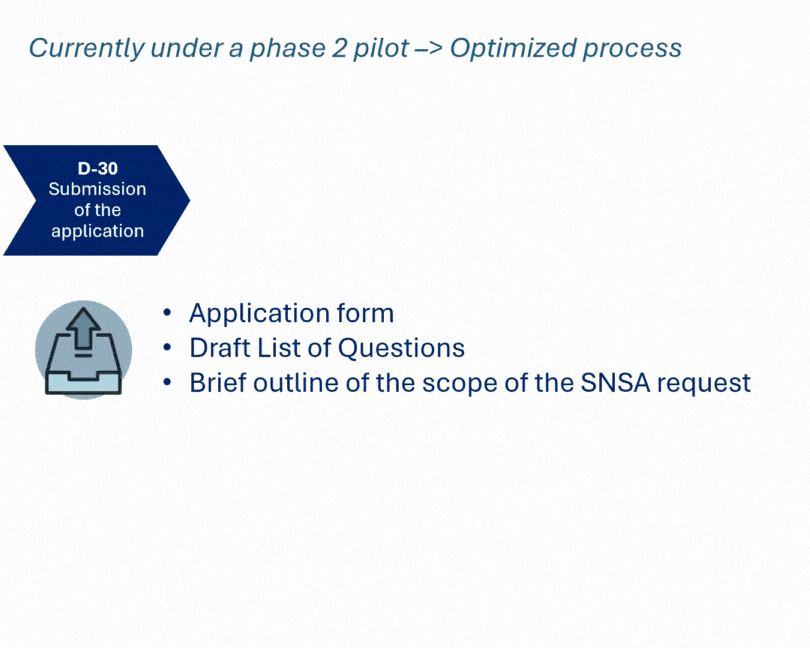
Developing a new medicinal product often involves navigating complex regulatory expectations. We recently supported a client through the SNSA procedure, engaging both Dutch and Belgian authorities in a joint scientific advice meeting.
This coordinated approach enabled a comprehensive review of CMC, non-clinical, and clinical aspects—particularly where existing guidelines lacked clarity. The outcome: a clearer, more confident development path for a complex product, aligned with regulatory expectations from the outset.
🔗 Learn more about SNSA
– Anne Walter
We had the pleasure of attending RAPS Euro Convergence 2024 in Brussels, where regulatory innovation and collaboration were at the heart of the discussions. Key topics included the upcoming EU pharma reform, access to medicines, and digital transformation. A valuable moment to connect with peers and reflect on the future of regulatory affairs.

- Sector Growth: 2,700 companies, 75,600 employees, +21% revenue growth, and €1.4 billion in R&D investments (+10%).
- Challenges: Despite high startup rates, business failures are increasing. Financing remains critical, with strong support from Bpifrance.
- Employment: Slight decline in 2024, but 83% of companies plan to hire in 2025 (+2,000 jobs).
- Tech Focus: Digital health and AI are booming, with 450 companies using AI and 76% in the commercialization phase.
- Government Support: €682 million allocated by Bpifrance, focusing on mental health and prevention. Tax incentives like CIR and JEI benefit 86% of companies.
- Sector Insights: Biotech focuses on oncology, neurology, and infectious diseases. Medtech faces MDR challenges. Digital health is advancing diagnostics and patient monitoring.
- Outlook: The sector is set for growth, driven by innovation, partnerships, and public support, though financing and regulatory compliance remain key hurdles.

The feedback session was a real success, thanks in particular to the presentation by our colleague Lorraine Balin. Her clear and insightful overview of the major regulatory developments provided valuable guidance on best practices and future directions for the pharmaceutical industry.
To extend the impact of this session, we are pleased to share a visual summary highlighting the key regulatory milestones discussed during the event. This infographic serves as a concise and educational tool to revisit the essential takeaways from a day rich in knowledge and exchange.
